Understanding actinide chemistry is critical for developing approaches for nuclear waste treatment as well as for illuminating the role of f orbitals in bonding and reactivity. A pair of studies describes new types of complexes these elements can form.
At the far end of the actinide series, the elements prefer to be divalent—nobelium(II), for example, is the only known stable state for that element in aqueous solution. Conversely, early actinides such as uranium, neptunium, and plutonium can adopt a number of oxidation states from+3 to +7 while typically avoiding the +2 state.
Four years ago, a team led by Matthew R. MacDonald and William J. Evans of the University of California, Irvine, prepared the first uranium(II) complex by reducing UIII[C5H4Si(CH3)3]3 with potassium graphite in the presence of 2.2.2-cryptand—an approach that also worked to synthesize several lanthanide(II) complexes.
Now, research teams have succeeded in synthesizing similar Np(II) and Pu(II) complexes (Chem. Sci. 2017, DOI: 10.1039/c7sc00034k and J. Am. Chem. Soc. 2017, DOI: 10.1021/jacs.7b00706)
“It is challenging to conceive of more difficult chemistry,” comments Thomas E. Albrecht-Schmitt of Florida State University. “In addition to tackling the high reactivity of these complexes, both teams had to contend with the very real radiologic issues associated with neptunium and plutonium.”

The neptunium work was led by Polly L. Arnold of the University of Edinburgh in collaboration with the European Union Joint Research Centre for transuranic science. The plutonium work was led by Andrew J. Gaunt and Stosh A. Kozimor of Los Alamos National Laboratory along with Evans and Filipp Furche of UC Irvine. Because of radiologic safety and nuclear security concerns, the chemists had to work with small amounts of materials, synthesizing less than 100 mg of the complexes.
Arnold and colleagues prepared several neptunium complexes in the +2, +3, and +4 oxidation states with cyclopentadienyl ligands. The complexes are generally more stable than their thorium or uranium analogs, and the researchers were able to determine that the ionic radius of neptunium shrinks by 8 picometers when it is oxidized from Np(III) to Np(IV). The product thought to be the {NpII[C5H4Si(CH3)3]3}1- complex, however, formed dark brown crystals that degraded too quickly to get X-ray diffraction data. Arnold’s lab has previously synthesized another Np(II) complex with a macrocyclic ligand, trans-calix[2]benzene[2]pyrrole.
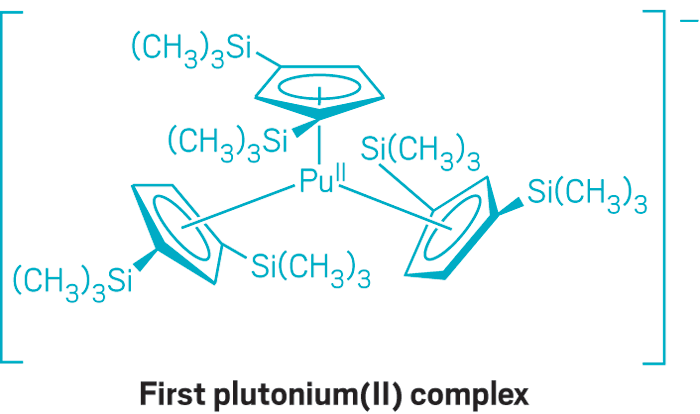
Gaunt and coworkers synthesized and structurally characterized the {PuII[C5H3(Si(CH3)3)2]3}1- complex, which forms purple crystals. The synthesis establishes plutonium as the actinide element with the largest number of confirmed oxidation states: 0 through +7, except +1.
Additional spectroscopic and theoretical analysis of the Pu(II) complex indicated that Pu(II) has a 5f6 ground-state electronic configuration, in contrast to d-orbital population observed for earlier actinides. The ground state for Th(II), for example, is 5f06d2, whereas the ground state for U(II) is 5f36d1. The results add to plutonium’s reputation for unique and complex properties that represent a transition point between the chemistry of lighter and heavier actinides.